Ever wondered what forms the very foundation of all matter? In this article, we journey into the microscopic world of atoms and molecules as we learn about the various types of chemical bonding.
Jump to:
What is Chemical Bonding?
Chemical bonding is the underlying process that forms molecules and compounds in the world of Chemistry. It is the attractive force between atoms that holds them together to create stable structures. Various bonding mechanisms can take place including ionic bonding, covalent bonding, metallic bonding, and hydrogen bonding.

Ionic Bonding
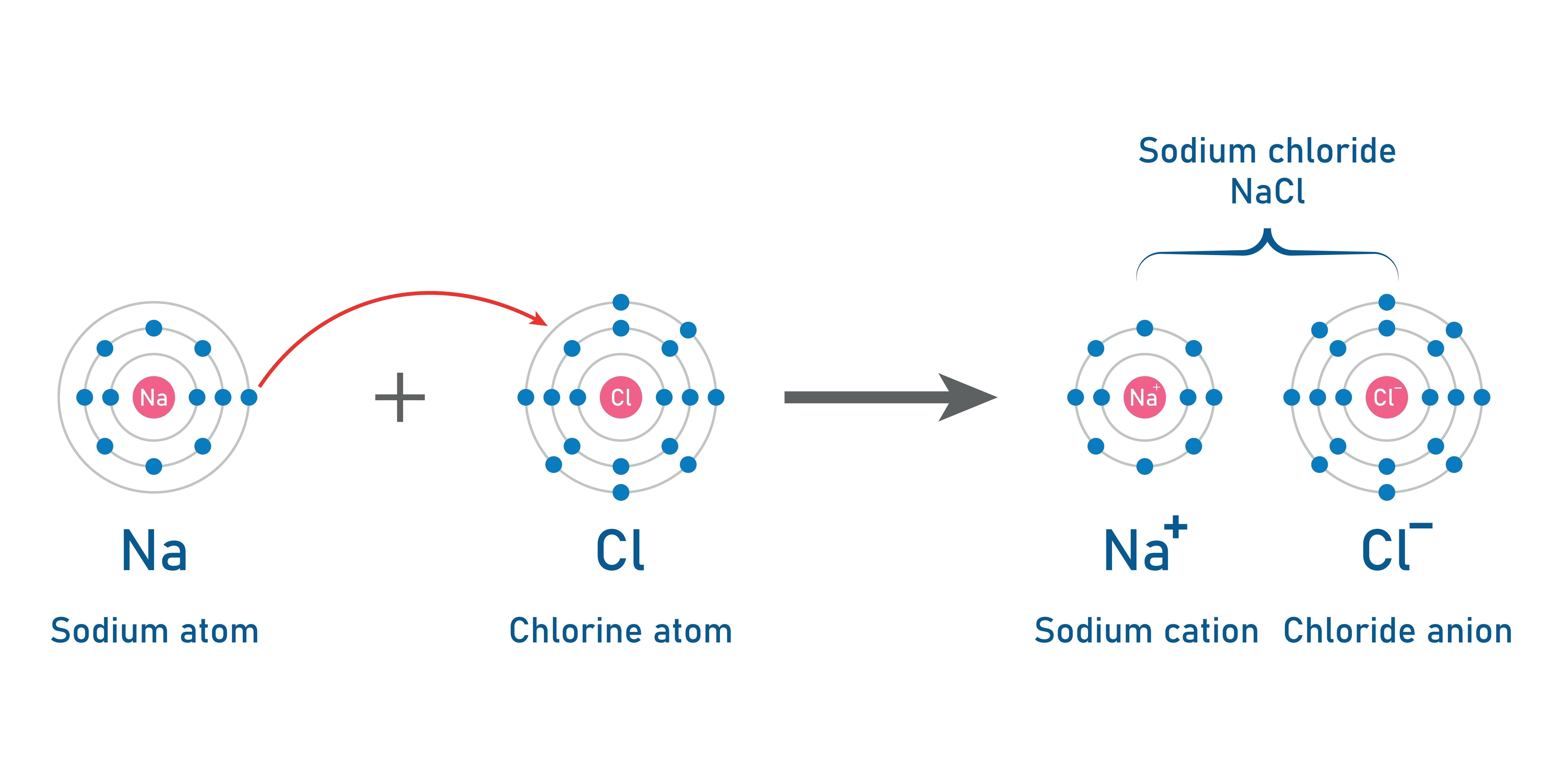
Ionic bonding is a type of chemical bonding that occurs between a metal and a nonmetal. In this process, electrons are transferred from the metal to the nonmetal, resulting in the formation of ions. The metal loses electrons to become a positively charged cation (a positively charged ion), while the nonmetal gains those electrons to become a negatively charged anion (ions that are negatively charged). The attraction between these oppositely charged ions leads to the creation of an ionic bond.
The process explained above is called ionisation or ion formation. Metals that are typically found on the left side of the periodic table tend to lose electrons and become positively charged ions. Whereas, nonmetals on the right-hand side of the periodic table gain electrons and form negatively charged ions.
Examples of compounds with Ionic bonds
Sodium Chloride (NaCl): Sodium (a metal), donates an electron to chlorine, (a nonmetal) forming Na⁺ and Cl⁻ ions. The resulting electrostatic attraction creates the familiar salt, sodium chloride.
Magnesium Oxide (MgO): Magnesium transfers electrons to oxygen, forming Mg²⁺ and O²⁻ ions. The combination of these ions results in the formation of magnesium oxide.
Potassium Bromide (KBr): Potassium gives up an electron to bromine, leading to the creation of K⁺ and Br⁻ ions. The resulting compound is potassium bromide.
Properties of Ionic compounds
- High melting and boiling points - Ionic compounds have high melting and boiling points due to the strong electrostatic forces between ions. Energy is required to overcome these forces and change the state of the compound.
- Solubility in water -Many ionic compounds are soluble in water, as water molecules surround and stabilise the separated ions, which facilitates their dispersal.
- Conductivity: In the molten or dissolved state, ionic compounds conduct electricity, as the ions are free to move and carry an electric charge.
- Crystalline structure: Ionic compounds often carry a crystalline structure. Ions arranged in a repeating pattern like this contribute to their stability.
Covalent Bonding
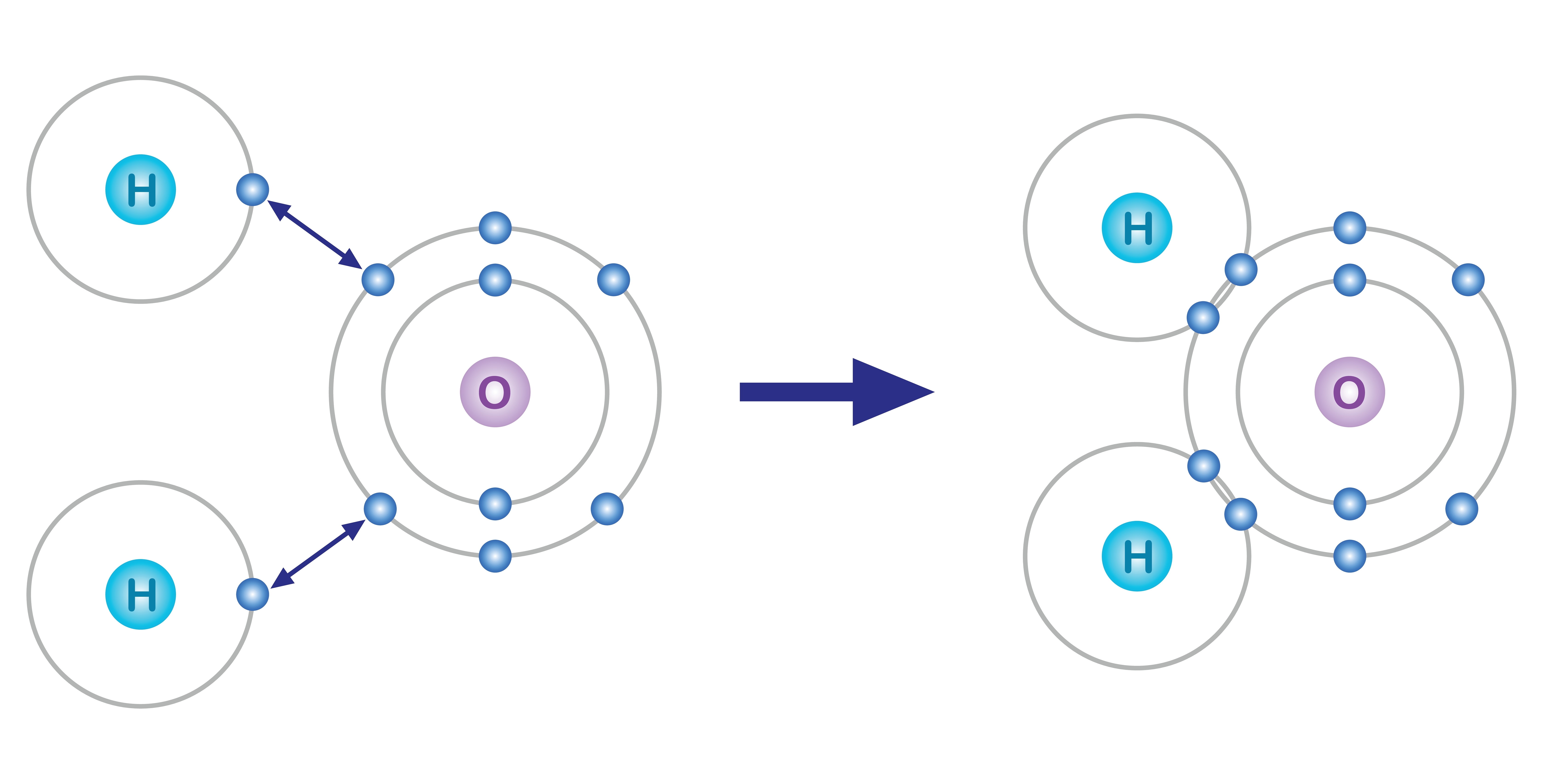
Covalent bonding occurs between two nonmetals. In contrast to ionic bonding where electrons are transferred, covalent bonding involves the sharing of electrons between atoms. In a covalent bond, atoms share one or more pairs of electrons, resulting in the formation of a molecule. This sharing of electrons allows both atoms to achieve a more stable electron configuration.
There are three types of covalent bonds; Single, Double, and Triple bonds. They essentially reflect the number of electron pairs shared between atoms and influence the strength and stability of the covalent bond. In a single covalent bond, two atoms share one pair of electrons. In a double bond, two atoms share two pairs of electrons and finally, in a triple covalent bond, two atoms share three pairs of electrons.
Examples of compounds with Covalent bonds
Water (H₂O): In water molecules, each hydrogen atom forms a single covalent bond with the oxygen atom, resulting in a distinctive bent molecular shape.
Methane (CH₄): Methane (a common hydrocarbon) is formed by four single covalent bonds between one carbon atom and four hydrogen atoms.
Carbon Dioxide (CO₂): Carbon dioxide features double covalent bonds between the carbon atom and each of the two oxygen atoms, contributing to its linear molecular structure.
Properties of Covalent compounds
- Low melting and boiling points - Due to the weaker forces holding the molecules together, covalent compounds generally have lower melting and boiling points compared to ionic compounds.
- Poor conductivity - Covalent compounds are typically poor conductors of electricity, as they don’t contain ions that can move to carry an electric charge.
- Varied Solubility- The solubility of covalent compounds varies. Some are soluble in water but others are not. This all depends on the nature of the molecules.
- Diverse Molecular Structures - Covalent compounds showcase a wide range of molecular structures, including linear, trigonal planar, tetrahedral, and more, based on the number of atoms and lone pairs around the central atom.
Metallic Bonding
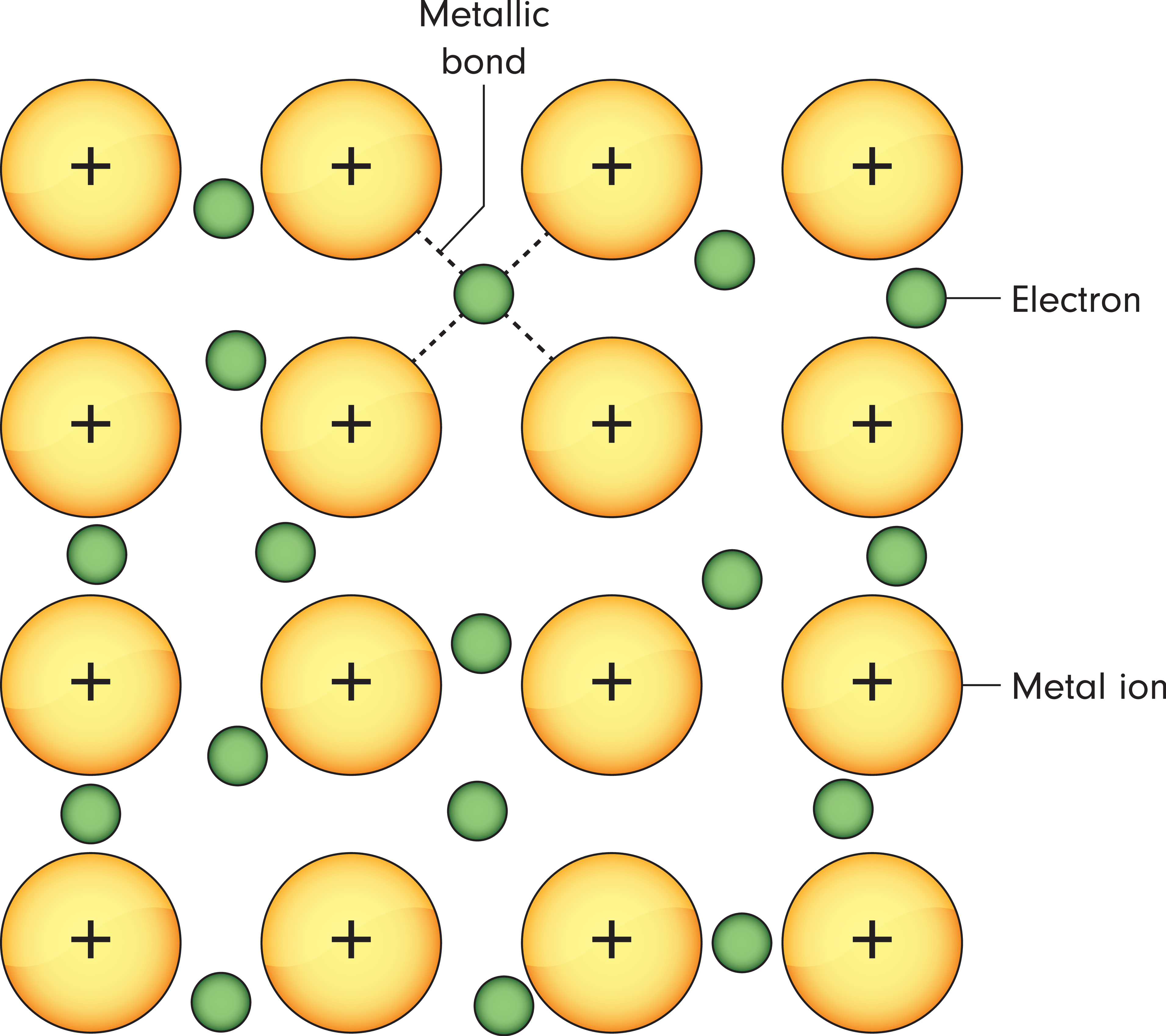
Metallic bonding is a unique type of chemical bonding that occurs between metal atoms. Unlike ionic or covalent bonds, metallic bonding involves a sea of delocalised electrons shared among positively charged metal ions. This electron "sea" allows for high electrical conductivity and gives metals their characteristic properties.
Metals, typically found on the left side of the periodic table, have one to three electrons in their outer shell, which they readily lose to form positively charged cations. These cations then arrange themselves in a regular, three-dimensional lattice, surrounded by a "sea" of mobile electrons.
Examples of metals with Metallic bonds
Copper (Cu): Copper is a great example of metallic bonding. In a copper sample, positively charged copper ions are arranged in a regular pattern, with the delocalised electrons moving freely between them.
Iron (Fe): Iron possesses the ability to conduct electricity, giving it its characteristic malleability and ductility.
Aluminium (Al): Aluminium is known for its lightweight yet strong properties, making it a crucial material in various industries.
Properties of Metallic compounds
- High electrical conductivity - The delocalised electrons in metallic bonds are free to move throughout the structure, allowing metals to conduct electricity effectively.
- Malleability and ductility - Metallic compounds are malleable (can be hammered into thin sheets) and ductile (can be drawn into wires) due to the ability of metal ions to slide past each other without breaking the metallic bonds.
- Lustre - Metals exhibit a lustre or shine due to the reflection of light by the delocalised electrons.
- High melting and boiling points - Metallic compounds generally have high melting and boiling points, as a significant amount of energy is required to break the strong metallic bonds.
- Thermal conductivity - Metals are excellent conductors of heat, as the delocalised electrons transfer thermal energy efficiently.
Recommended for you!
Best SellersHydrogen Bonding
Hydrogen bonding is a specific type of intermolecular force that occurs when a hydrogen atom, already covalently bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine), interacts with another electronegative atom nearby. This interaction results in a strong, partially electrostatic bond known as a hydrogen bond.
There are certain conditions required for Hydrogen bonding:
Hydrogen donor and acceptor: There must be a hydrogen atom covalently bonded to a highly electronegative atom, acting as the hydrogen bond donor. The neighbouring molecule must have an electronegative atom capable of accepting the hydrogen bond.
Directionality: Hydrogen bonds exhibit directionality, meaning they form in a linear or near-linear arrangement. This results in a stronger bond compared to non-directional forces.
Examples of compounds with Hydrogen bonds
Water (H₂O): Water is a classic example of hydrogen bonding. The hydrogen atoms in one water molecule form hydrogen bonds with the oxygen atoms in neighbouring water molecules, creating a network of interconnected molecules.
Ammonia (NH₃): In ammonia, the hydrogen atom is covalently bonded to nitrogen, and the lone pair on nitrogen can form a hydrogen bond with another electronegative atom.
Hydrogen Fluoride (HF): In Hydrogen fluoride the hydrogen atom is bonded to fluorine, and the fluorine atom accepts a hydrogen bond from another molecule.
The specificity and strength of hydrogen bonds significantly contribute to the structure and function of biomolecules. That's why understanding hydrogen bonding is vital for learning about the behaviour of molecules in biological systems.
If you’re keen to jump further into the world of Chemistry, we’d recommend exploring our accredited Chemistry Diploma Course available for just £29 (save £118!). It includes an entire module on chemical bonding where you’ll be introduced to intermolecular forces and bond polarity. This course also covers essential modules such as electrochemistry, hydrocarbons, transition elements, and more. Expand your knowledge and ignite your passion for chemistry today!